
Radiation in the ocean and Fukushima
Global Research Report
Geiger counters in the hands of citizens discovered 150 CPM coming from the ocean.
Let’s begin by doing the mathematical on what 150 CPM (counts per minute) indicates. One Bq is 1 decay event per second. So split CPM by 60, and you have Bq found. This article found 2.5 Bq. But in your body, on a regular day, you have 4,400 Bq. See: Wikipedia on Bequerel.
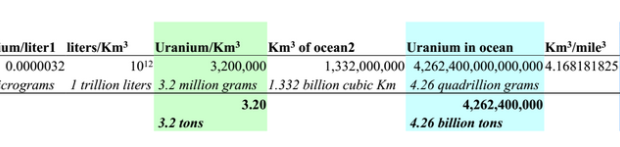
So why is there radiation found near the ocean, and 5 times what’s on land? Most people have no idea that there are 3.2 tons of natural uranium in every cubic kilometer of ocean. (13.34 tons per cubic mile.) The ocean has a total of 4.2 billion tons of uranium in it. So, yeah. When you go near the ocean, you find radiation. But it’s always been there. There’s also potassium-40, radium, polonium-210. Like the spaghetti sauce, you name it, it’s in there. And it’s perfectly fine. It’s in your sea-salt.
But let’s concentrate on uranium, so we can get a sense for natural radiation sources.
San Franciso Bay is 400-1600 square miles depending on what part is used. For this calculation 1000 square miles will be used.
Depth of San Francisco Bay varies, but its average is 15 feet deep.
15 ft ÷ 5280ft/mile = 0.00284 miles deep.
0.00284 x 1000 square miles = 2.84 cubic miles of seawater.
Seawater averages 13.34 tons of natural uranium per cubic mile.
2.84 cubic miles x 13.34 metric tons uranium/cubic mile = 37.8977 metric tons of uranium
Using 2204.622 lbs per metric ton, there are 83550.16 lbs of uranium in San Francisco Bay.
0.72% of natural uranium is U-235, suitable for making bombs. (Ignoring U-234)
0.0072 x 83550.16 lbs = 601.56 lbs of U-235 in San Francisco Bay.
It took 140 lbs of 80% enriched uranium to make the Hiroshima atomic bomb.
Since that U-235 wasn’t pure, we need to calculate how much 601.56 lbs of pure U-235 would be at 80% enrichment.
601.56 lb of U-235 ÷ 0.80 = 751.95 lbs of 80% enriched uranium.
751.95 lbs ÷ 140 lbs per bomb = 5.371 bombs.
On a normal day, San Francisco Bay has enough U-235 dissolved into it to build at least 5 Hiroshima sized bombs.
And yet, because it is dissolved in the ocean, you can swim in it. It is safe. You can eat the fish.The ocean is big. Even a bay is huge.
So what’s coming from Fukushima?
The average person hears Bq numbers, and doesn’t have the background to translate them into meaning.There are also wild exaggerations out, mostly from RT and other Russian sources. And most of the public is totally unaware that Russia has reaped a huge (global warming producing) bonanza from Japan’s shutdown of nuclear power plants.
That’s because Russia is selling oil and natural gas to Japan to replace Japan’s nuclear power. Russia wants that to continue – global warming be damned. I was in Russia talking about the factories in Siberia 10 years ago.
Those factories that produce most of the world’s (now mostly illegal) freon for refrigeration. Freon and other chlorinated fluorocarbons are a major global warming and ozone depletion gas that most of the world has decided is very bad for the environment. And I realized that for a guy in Siberia looking out the window at the snow and permafrost, the idea of warming things up sounds pretty good.
So there’s that Russian conflict of interest to think about, and there’s a general interest in Russia in warming things up to open Siberia. Russian oil men are not much different from Texas oil men, or British Petroleum oil men, or Saudi princes. They want to pump oil and gas and sell it.
Reported TEPCO figures are 20-40 trillion Bequerels. RT published 15 quadrillion Bq (15,000 billion) as escaping from Fukushima. It’s a wild exaggeration, but, let’s use it anyway, because it shows us the same thing.
Measuring radiation in Bq is like measuring sugar in the kitchen by the molecule. So let’s ask ourselves how much sugar would it be, if every Bq (or decay) per second was sugar. Sugar is 342 grams per molecular weight. A molecular weight has a fixed (and outrageously large) number of molecules in it. (Avogadro’s number). Atomic weight is what is used to figure out molecular weights and count atoms.
Avogadro’s number is 602,214,130,000,000,000,000,000
So now we will divide RT’s 15 quadrillion sugar molecules by Avogadro’s number.
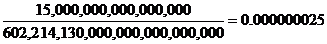
Multiply 25 billionths of a molecular weight by 342 grams to get the number of grams of sugar.
0.000000025 x 342 grams = 0.0000085 grams
1 grain of sugar is about 100 milligrams.
You would have to break 1 grain of sugar into 11,000 pieces, and take one of them to put your finger on 15 quadrillion sugar molecules.
In other words – you couldn’t find it on your kitchen counter without a magnifying glass.
And that’s the wild exaggeration version of what has gone into the ocean from Fukushima.
Yes, we can detect atoms of Cs-137 in modern laboratories because we have phenomenally sensitive equipment that can notice single atoms. But atoms are extremely small.
You cannot detect that radioactivity from Fukushima with your Geiger counter. If you think that you are detecting it, you are saying that you can detect the equivalent of 1 grain of sugar broken into 11,000 pieces, where just one of those pieces went into the Pacific ocean on the coast of Japan. You are saying that up against that tiny amount, you can find that in the ocean, when San Francisco Bay alone has enough U-235 in it to make 5 atomic bombs, and corresponding amounts of ocean just off the coast have the same.
I think it’s great that people have Geiger counters and get into it. But you have to learn more about what’s really going on in order to make sense out of it.
Radiation is everywhere, always. You can’t get away from it. And that’s just fine. Enjoy the ocean. It’s always had tons of radioactive stuff in it. Always will.
http://www.lbl.gov/abc/wallchart/chapters/15/3.html
http://darchive.mblwhoilibrary.org
http://en.wikipedia.org/wiki/San_Francisco_Bay
Brian Hanley, PhD Butterfly Sciences Davis -
- See more at: http://globalresearchreport.com/2013/12/31/radiation-in-the-ocean-and-fukushima/?utm_source=feedburner&utm_medium=email&utm_campaign=Feed%3A+globalresearchreport+(Global+Research+Report)#sthash.aMccsGje.dpuf
